The Pharmaceutical Society of Uganda (PSU), in collaboration with partners in Uganda, Democratic Republic of Congo (DRC) and South Sudan, was awarded a highly competitive research and innovation grant by the International Centre for Insect Physiology and Ecology (icipe) under the BioInnovate Africa programme. The project is titled “Development of Biobased Supplements to Artemisinin Combination Therapy for Effective Malaria Treatment (SUPPACT).”
The need for novel solutions for malaria
There is a resurgence of malaria in Africa due to declining efficacy of artemisinin combination therapies (ACTs) used as first-line treatment. The loss of ACT efficacy arises from widespread mutation-induced resistance by plasmodium species, the causative agent for malaria. Quinine, used as a second-line treatment for malaria, is associated with serious adverse effects. There are also reports of varying levels of resistance to sulfadoxine and pyrimethamine (SP), the drugs used for seasonal malaria chemoprevention and intermittent preventative treatment in pregnancy. Vector control strategies aimed at eradicating the female Anopheles mosquitoes that spread the Plasmodium parasites, or preventing their bites through use of insecticide-treated mosquito nets, are being hampered by widespread resistance against pyrethroids.
However, malaria remains a significant health challenge globally, particularly in sub-Saharan Africa with 95% of malaria cases and 96% of malaria deaths occurring in 2020 and about 80% of them being children under 5 years of age. The region of East Africa Community (EAC), which is now home to over 470 million people in seven countries (Kenya, Uganda, Tanzania, Rwanda, Burundi, Democratic Republic of Congo and South Sudan), is highly burdened by the disease. A recent study in three EAC countries estimated mean parasite prevalence at 4.7% in Kenya, 10.6% in mainland Tanzania, and 9.5% in Uganda. Assuming that similar trends obtain in Rwanda, Burundi, DRC and South Sudan, the number of people with malaria at any point could be as high as 39 million.
The proposed innovation
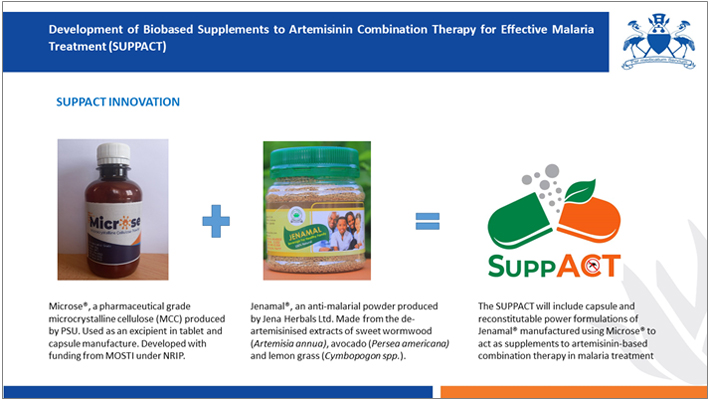
The SUPPACT project builds on the Pharmaceutical Society of Uganda’s (PSU) recent work on microcrystalline cellulose (Microse®) which was supported by MoSTI under NRIP in 2021/2022. Leveraging on Jena Herbals Ltd’s extensive research on a deartemisinised formulation of Artemisia species (Jenamal®), which has been demonstrated to possess significant efficacy in preventing resistant malaria in animal models, the project team seeks develop standardized and clinically evaluated solid dosage formulations capable of augmenting ACTs in malaria treatment.
Objectives of the Project
Main Objective
The project’s main objective is to develop and standardize non-artemisinin components of Artemisia species into microcrystalline cellulose-based phytopharmaceuticals with enhanced product safety, efficacy, quality, and bioavailability for supplementation of ACTs in the treatment of malaria and reversal of parasite resistance.
Specific Objectives
Specifically, the project will;
- Identify and chemically profile deartemisinised active and non-active compounds in Artemisia species with synergistic actions to ACTs against sensitive and resistant malaria parasites in vitro.
- Optimize the effective and safe doses in vivo of the chemically profiled compounds as a basis of their co-formulation as a supplement to ACTs in treating malaria.
- Co-formulate the active and non-active compounds into phytopharmaceutical dosage forms using pharmaceutical-grade microcrystalline cellulose from maize cobs.
- Study the safety and efficacy of the co-formulated phytopharmaceutical dosage forms when administered as supplements to ACTs in the treatment of malaria.
- Compile a product dossier to attain regulatory approvals as a prerequisite for application for marketing authorization and commercialisation in the region.
- Preserve the best-yielding plant species for the sustainability of quality raw materials.
Expected project outputs
The project will develop two market ready solid dosage forms, i.e., capsules for adults and a reconstitutable powder for children, each with a dossier to support application for market authorization. A total of 4 graduate students will be trained and value chain actors (including farmers of maize and Artemisinin species) sensitized. Through stakeholder engagements, a policy framework will be developed to ensure mainstream adoption of SUPPACT as a supplement to ACTs in routine management of malaria. The project outcomes will include both health and economic impacts in form of reduced morbidity, mortality and cost of treatment, as well as increased jobs, value addition, import substitution and productivity of the population.
Outcomes
By the end of the project, the project team anticipates to achieve the following deliverables:
- Malaria patients to use SUPPACT as part of the ACT treatment regimen.
- Healthcare providers to prescribe SUPPACT in combination wth ACTs in malaria treatment.
- Ministries of Health of participating countries to include SUPPACT in the national malaria treatment guidelines.
In turn, these will culminate into reduced resistance to ACTs in the population using SUPPACT as an adjunct to ACT in malaria treatment.
Funding Organisation:
International Centre of Insect Physiology and Ecology (icipe)
P.O. Box 30772-00100 Nairobi, Kenya.
Website: http://www.icipe.org/

Project Partners:




SUPPACT Project Team
The Project Leader is Dr. Jonans Tusiimire, the Principal Investigator for SUPPACT. He will coordinate the PSU team in the production of Microse® as well as SUPPACT quality control analysis, quality assurance processes, regulatory compliance and stakeholder engagements.
The Jena Herbals Ltd team is led by Assoc. Prof. Patrick Ogwang Engeu. He will lead product innovation, efficacy and safety evaluation in animal models, pilot production of SUPPACT for clinical trials, and commercialization plans.
The University of Bukavu team is led by Dr. Mushagalusa Kasali Félicien. He will lead studies in extract preparation, chemical compound profiling, isolation, and structural elucidation, and phase 2a & 2b clinical trials.
The University of Bahr el Ghazal team is led by Dr. Lina Sara Mathew Alonga. She will be responsible for Artemisia species identification and authentication, cultivation and conservation in botanical gardens, raw material collection, primary processing and quality control.
